Oxygen reduction reaction (ORR) is a key step in H2-O2 fuel cell, which is however facings slow kinetics. Platinum is acknowledged to be the best catalyst for the ORR, however, their scarcity and high cost remain one of the greatest obstacles to the large-scale commercialization of H2-O2 Fuel Cell. To address this issue, enormous efforts have been devoted to developing novel catalytic materials, like low platinum alloys or core-shell structured platinum catalysts and non-platinum heteroatom-doped carbon catalysts. However, the overpotential of ORR needs to be reduced at least ca. 0.2 V even for the state-of-the-art catalyst.
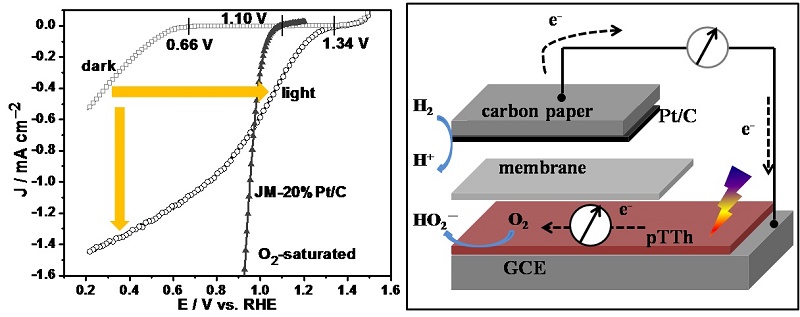
Our group developed a new strategy, by taking the advantage of photocatalysis, to accelerate the ORR. By coupling the photocatalysis, the kinetics of the ORR is significantly accelerated with a polymer semiconductor catalyst (e.g., polyterthiophene). The onset potential shows a positive shift from 0.66 to 1.34 V, and the current is enhanced by 44 times at 0.6 V. The improvement is further demonstrated with a proof-of-concept light-driven H2-O2 fuel cell, in which the open circuit voltage increases from 0.64 to 1.18 V and the short circuit current is doubled. The increased in the potential, conferred by illumination, is respectively to be 0.68 and 0.54 V for the half-cell and fuel cell, and such an increase is close to the photovoltage of polymer solar cells, which were reported to be in the range of 0.6-0.8 V. It is, therefore, concluded that this novel light-driven fuel cell is the tandem structure of polymer solar cell and fuel cell, which enables the utilization of both photo- and electrochemical energy simultaneously, showing a promise in energy conversion and storage.
This work ware recently published in the Angewandt Chemie International Edition (DOI:10.1002/anie.201607118 ) This work was financially supported by 973 National Basic Research Program of the Ministry of Science and Technology (grant 2014CB239400), the National Natural Science Foundation of China (no. 1090340) and iChEM. (Text and photo by Bingqing ZHANG).