Recently, research group from Dalian Institute of Chemical Physics (DICP) and Dalian National Laboratory for Clean Energy (DNL) unraveled a single-step two-electron transfer mechanism from photo-irradiated CdS to molecular catalyst CoPy in CoPy/CdS hybrid photocatalytic systems under strong alkaline conditions. This work has been published recently as a Communication in Journal of the American Chemical Society ( DOI: 10.1021/jacs.6b04080).
The group has been working on the hybrid photocatalytic systems comprised of semiconductors and molecular catalysts for water reduction for many years (J. Catal., 2011, 281, 318; ChemSusChem, 2012, 5, 849; Chem. Commun., 2012, 48, 988; Acc. Chem. Res., 2013, 46, 2355; J. Catal., 2016, 338, 168). It was realized that the matching of the energy levels between semiconductor and molecular catalyst is a crucial factor to be considered for the construction of such hybrid systems. For most of the solar fuel production reactions, multi-electron transfer from light harvester to catalyst or reactant is necessary. Therefore, better understanding the charge transfer mechanism (one-electron transfer or multi-electron transfer) between semiconductor and molecular catalyst in photocatalytic system is important for the designing, synthesis and assembly of more efficient photocatalytic systems for water splitting and CO2 reduction.
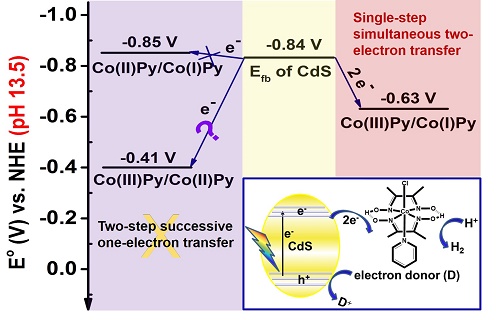
In this work, the electron transfer processes in Co(III)Py/CdS hybrid system under different pH conditions have been investigated in detail. Energy level analysis indicates that two-step successive one-electron transfer from CdS to Co(III)Py to yield the key proton reduction intermediate Co(I)Py species under high pH conditions (pH 13.5) is thermodynamically forbidden. However, enhanced photocatalytic H2 evolution activity was indeed observed at pH 13.5. Charge transfer dynamics and kinetics studies showed that the single-step simultaneous two-electron transfer from CdS to Co(III)Pyto yield the reduced Co(I)Py species is the most plausible mechanism accounting for the enabling of the photocatalytic H2 evolution activity at pH 13.5.
In terms of the driving force, single-step simultaneous multi-electron transfer process is energetically more favorable than successive multi-electron transfer process according to Hess’s law. Therefore, revelation of single-step simultaneous two-electron transfer processes from semiconductor to molecular catalyst is very important for the designing of more efficient semiconductor-molecular catalyst hybrid system and optimization of the photocatalytic reaction conditions.
The first author of this work is PhD candidate Yuxing Xu, co-supervised by Prof. Hongxian Han and Prof. Can Li. This work has been financially supported by National Natural Science Foundation of China, 973 National Basic Research Program of the Ministry of Science and Technology,and the Collaborative Innovation Center of Chemistry for Energy Materials. (Reported by Hongxian Han, Yuxing Xu).