Hydrogen sulfide splitting into hydrogen and sulfur through an off-field electrocatalysis
Zijin Wang‡, Qing-Nan Wang‡, Weiguang Ma, Tiefeng Liu, Wei Zhang, Panwang Zhou, Mingrun Li, Xinyi Liu, Qingbo Chang, Haibing Zheng, Ben Chang, and Can Li*
https://pubs.acs.org/doi/10.1021/acs.est.4c00312
Hydrogen sulfide (H2S), a toxic gas abundant in natural gas fields and refineries, is currently removed mainly via the Claus process. However, the emission of sulfur-containing pollutants is hard to be prevented and hydrogen element is combined to water. Herein, we report an electron-mediated off-field electrocatalysis approach (OFEC©) for complete splitting of H2S into H2 and S at ambient conditions. Fe(III)/Fe(II) and V(II)/V(III) redox mediators are used to fulfil the cycles for H2S oxidation and H2 production, respectively. Fe(III) effectively removes H2S with an almost 100% conversion during its oxidation process. The H+ ions are reduced by V(II) on non-precious metal catalyst, tungsten carbide. The mediators are regenerated in an electrolyzer at a cell voltage of 1.05 V, close to the theoretical potential difference (1.02 V) between Fe(III)/Fe(II) and V(II)/V(III). In a laboratory bench-scale plant, the energy consumption for the production of H2 from H2S is estimated to be 2.8 kWh Nm−3 H2 using Fe(III)/Fe(II) and V(II)/V(III) mediators, and further reduced to about 0.5 kWh Nm−3 H2 when employing well-designed heteropolyacid/quinone mediators. The OFEC© presents a cost-effective approach for the simultaneous production of H2 and elemental sulfur from H2S, along with complete removal of H2S from industrial processes. It also provides a practical platform for electrochemical reactions involving solid precipitation and organic synthesis.
我组研发成功了离场电催化新技术,在室温、常压下实现硫化氢全分解制氢和硫磺,有望替代工业现行的克劳斯技术,实现天然气开采、炼油化工行业和煤化工过程中硫化氢的消除和资源化利用,并成为低成本制绿氢的一个新路径。
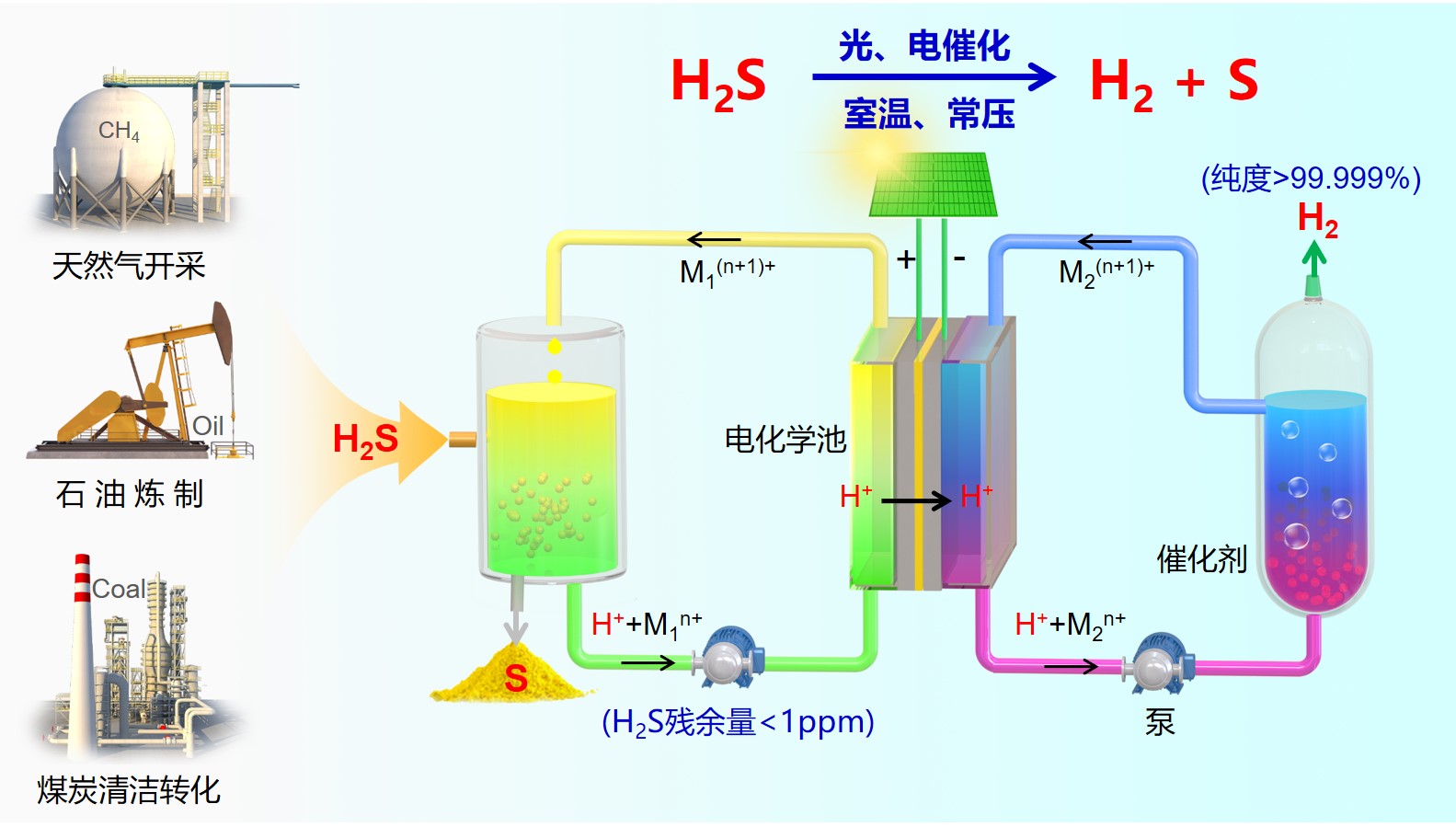
硫化氢是一种有害化合物,又是一种重要的资源,主要伴生或副产于天然气开采、炼油化工行业和煤化工过程。据不完全统计,我国每年的硫化氢处理量至80亿立方米,潜在的待处理量超过100亿立方米,全球范围每年处理量超过700亿立方米。硫化氢消除并资源化利用是天然气开采、炼油化工行业、煤化工等工业过程中长期面临的难题,也是化学工业中具有百年历史的重要难题。现行的克劳斯工艺通过将硫化氢氧化成硫磺和水而消除,但该过程排放大量的含硫污染物,需要进一步二次处理,即使经过多步克劳斯过程,尾气中含硫污染物也不能被完全消除。此外,上述过程只回收了硫化氢中的硫磺,其氢元素被转化为水而损失。
我组早在2003年就开始致力于采用非常规技术进行硫化氢分解反应的研究,先后采用光催化 (J. Catal., 2008, 260, 134;Chi. J. Catal., 2008, 29, 313)、电催化 (Angew. Chem. Int. Ed., 2014, 53, 4399; Angew. Chem. Int. Ed., 2018, 57, 3473)、光电催化 (ACS Catal., 2016, 6, 6198; ACS Energy Lett. 2020, 5, 2, 597) 等技术探索了硫化氢分解制氢和硫磺,原理上验证了光电催化技术路线的可行性。
在前期工作的基础上,我组解决了规模化分解硫化氢工程放大问题,通过电子介导对,将化学反应和电极表面的电荷交换反应解耦,并利用现代化工反应器,将氧化反应(硫磺生成)和还原反应(放氢过程)转移并离开电极,开发了离场电催化技术,在电化学池外部连结釜式反应器和悬浮床/固定床反应器分别实现硫化氢分解制硫磺和放氢反应,而在电化学池内实现了电子介导对的再生。我组已经完成了实验室100升硫化氢/天的小试规模的技术验证和长周期运行实验,硫化氢转化率可大于99.9999%,含硫污染物排放低于1 ppm (百万分之一),氢气纯度达到99.999%以上。该离场电催化反应模式原理上解决了电化学技术放大的工程难题。该项技术已申请了17项专利,7项授权,组成了完整的专利包,形成了具有我国自主知识产权的原创性技术。我组已经和天然气开采、石油化工炼制、煤化工相关企业合作,正在推进硫化氢完全分解制氢和硫磺项目的工业化放大试验。
相关工作以“Hydrogen sulfide splitting into hydrogen and sulfur through an off-field electrocatalysis”为题,于近日发表在Environmental Science & Technology上。审稿人认为“The off-field electrocatalysis technology presented therein represents a significant advancement in the field of H2S splitting into H2 and sulfur, offering a promising and innovative approach to compete with the current Clause technology”以及“This strategy absolutely prevents the pollution of sulfur/sulfide from the anode, membrane, and cathodic electrolyte”。该工作的共同第一作者是DNL1600组群博士研究生王子衿和博士后王庆楠博士,前期贡献者有博士后马伟光博士等人。上述工作得到了国家自然科学基金委“人工光合成”基础科学中心等项目和企业的支持。(文/图 王庆楠、王子衿)