Recently, the Solar Energy Team in DNL led by Prof. Can Li, in collaboration with Prof. Lianzhou Wang’s research group of The University of Queensland of Australia achieved the overall splitting of Hydrogen Sulfide (H2S) using an innovative photoelectrochemical–chemical loop reaction system. This work was published in Angewandte Chemie International Edition as a ‘hot paper’ (Angew. Chem. Int. Ed. 2014, doi: /10.1002/anie.201400571 ).
Hydrogen sulfide is an abundant chemical existed in nature and also produced from chemical industry as a by-product. Although H2S represents potential resources of two elements, S and H2 which are individually of valued chemicals, its economic potential has not been well recognized because of its extremely toxic property. Currently, H2S is mainly treated with Claus process wherein it is partially oxidized to water and elemental S, namely, the hydrogen element is not recovered. Therefore, it is highly desirable to develop a cost-effective process to simultaneously recover H2 and S from H2S.
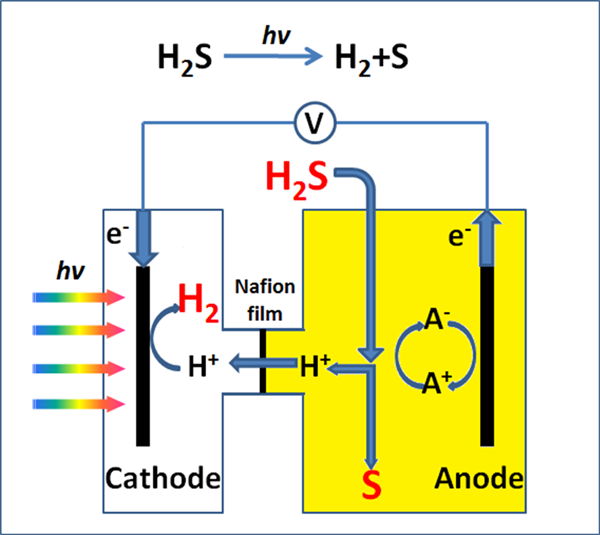
In this work, an integrated photoelectrochemical–chemical loop for solar-driven overall splitting of H?2S is constructed. This process of converting H2S to H2 and S consists of two integrated reactions. The first reaction is a simple chemical reaction that can efficiently trap and selectively convert H2S to S and protons by the redox couples (I3-/I- or Fe3+/Fe2+). The second reaction is a photoelectrochemical reaction that can reduce protons to generate H2. Meanwhile, the reduction state of the redox couples is restored to the initial oxidation state by the photogenerated holes. Thus with the link of the redox couples, the net reaction is the overall splitting of H2S to produce both H2 and S using solar energy.