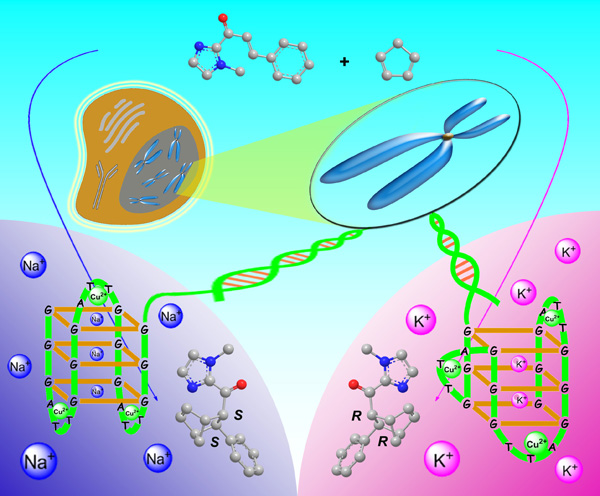
We found that G-quadruplex DNA (G4DNA) shows enantioselective catalytic performance in the Diels-Alder reaction, and the research results were recently published in the journal of Chem. Commun. (DOI: 10.1039/ C3CC45396K) and this paper was selected as a ‘hot paper’ in current issue.
As is known to all, alkali metal ions Na+ and K+ play important roles in a wide variety of biological processes, such as Na+/K+-ATPase (also known as the Na+/K+ pump). In nature, Na+ and K+ serve as cofactors and structural roles in assisting the function of biologically active macromolecules such as protein enzymes and nucleic acids. When binding to DNA, Na+ and K+ are well known for mediating formation and stabilization of DNA G-quadruplexes, such as an antiparallel G4DNA is formed in Na+-containing solution while a hybrid-type G4DNA is adopted in K+-containing solution. Very recently, DNA G-quadruplex structures have been visualized in human cells during cell-cycle progression, suggesting that nature has provided the opportunities that G4DNA might function as a catalytic species such as RNA in biological processes. Although DNA G-quadruplexes have provided diverse chiral scaffolds in the presence of alkali metal ions such as Na+ and K+, the enantioselective catalytic functions of G4DNA in Na+ and K+ media are still largely elusive.
In this work, we report that the enantioselectivity can be sensitively tuned by changing the alkali metal ions in the enantioselective Diels-Alder reaction catalyzed by a G4DNA-based catalyst. Interestingly, the enantiomeric excess of the Diels-Alder reaction can be switched from 49% to 56% by just changing Na+ to K+, which is attributed to the structural transformation of G4DNA from antiparallel to hybrid-type. By tuning the ratio of Na+/K+, the enantioselectivity of the Diels-Alder reaction could be switchable and shows much more sensitive to K+ than to Na+.
Followed by our previous reports in G4DNA-based enantioselective catalysis (Angew. Chem. Int. Ed. 2012, 51, 9352-9355 and Chem. Commun. 2012, 48, 6232-6234), these results could be helpful for understanding the recognition of the chiral compounds (possibly drug molecules) to the potential G-quadruplex targets for cancer therapy and may shed light on the chemical behaviours of DNA in the life process.
This work was financially supported by the National Science Foundation of China (Grant Number 31000392).
Article
Changhao Wang, Guoqing Jia, Yinghao Li, Sufang Zhang and Can Li*
Chem. Commun., 2013, DOI: 10.1039/C3CC45396K
http://pubs.rsc.org/en/content/articlelanding/2013/cc/c3cc45396k#divAbstract