Recently, our group made new progress in heterogeneous asymmetric catalysis. We developed the first example about bifunctional chiral base catalyzed addition of thiols to in situ generated aryl- and alkyl- substituted ortho-Quinone methides (o-QMs) in excellent reactivities and enantioselectivities. This study provides a new synthetic method to access bioactive interesting a-aryl- and a-alkyl-substituted benzyl mercaptans. The research paper has been published online in Angew.Chem.Int.Ed., doi: 10.1002/anie.201409894, http://dx.doi.org/10.1002/anie.201409894 .
Heterogeneous asymmetric catalysis is one of the efficient methods for the preparation of chiral compounds. Due to its combination of basic science research and potential industrial applications, heterogeneous chiral catalysts or reaction systems with high reactivities and selectivities are extremely demand. Our group has been devoting to the development of new heterogeneous catalytic systems. In this area, we reported the first example about grafting Sharpless catalyst onto the surface of silica and in the mesopores of MCM-41(Angew. Chem. Int. Ed., 2002, 821).In addition, during of studying chiral catalysis in nanopores, we observed the molecular [Co(salen)] catalyst confined in SBA-16 show a significantly enhanced cooperative activation effect in the hydrolytic kinetic resolution of epoxides (Angew. Chem. Int. Ed., 2007, 6861) and the enhancement of the performance confined within carbon nanotubes (Angew. Chem. Int. Ed., 2011, 4913). Furthermore, we also conducted asymmetric catalysis reactions in liquid-liquid biphasic systems and posed the concept of “enmulsion catalysis” in academia (Chem. Eur. J.,2004, 2277). Recently, a series of Aldol reactions have been published by utilizing the concept of “emulsion catalysis” (J. Catal., 2007, 360; Green Chem., 2011, 1983; Angew. Chem. Int. Ed., 2012, 13159).
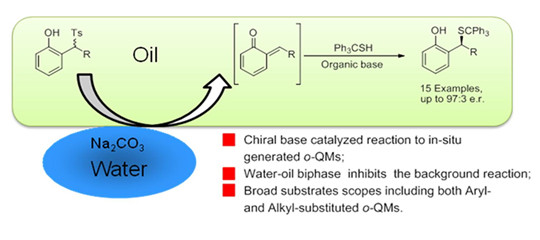
Ortho-Quinone methides (o-QMs) are important intermediates in a number of biological processes, and have been identified as active species in many chemical reactions. Despite their broad synthetic utility, only very few catalytic systems have been reported to date on the enantioselective addition to o-QMs. To our best knowledge, no catalytic system based on a chiral organic base has been reported for the reaction involving o-QMs. This may be attributed to the fact that reactive o-QMs are often generated in situ from various precursors with the assistance of either acid or base, which is not compatible with catalysts based on organic bases or may provide racemic products as a result of a significant background reaction. This work further exploits the advantage of water-oil biphase in asymmetric catalysis. A water-oil biphasic reaction system was developed to realize the spatial separation between a) the organic-base catalyst and reactants in the organic phase, and b) the inorganic base in the aqueous phase, and thus to suppress the racemic background reaction. Based on this method, an acid-base bifunctional organic catalyst achieved excellent chiral induction and extended substrate scope in the asymmetric reaction of the o-QMs. This study paves a new route for the reactions involving o-QMs and water-oil biphasic system.
This work is financially supported by National Natural Science Foundation of China, the Basic Research Program of China (973 project) and the National Science Fund for Excellent Young Scholars. (by Yan Liu and Wengang Guo)